Regulatory Updates
- Todo
- Biocides
- Carbon Footprint
- CLP
- Cosméticos
- Cosmetics
- Detergents
- Ecolabel
- Environment
- GB CLP
- KKDIK
- Packaging
- PFAS
- PIC
- REACH
- UA-REACH
- UK-REACH
- Waste

The BPC supports the approval of ethanol under the BPR
The BC supports the approval of ethanol. After several meetings, it remains pending a final conclusion on whether it has carcinogenic properties, etc.

One in Five Hazardous Products are not notified to Poison Centres in Europe
According to the latest ECHA’s inspection, one in Five Hazardous Products according to CLP are not notified to Poison Centres in Europe.

UK REACH Deadlines Extended
UK REACH Deadlines Extended: DEFRA Publishes Consultation Response and the new period for registration will start in 2029.
New Poison Centre obligations in Belgium and Switzerland from 2026
New Poison Centre obligations in Belgium and Switzerland from 2026. Key points for hazardous mixture notification under CLP and ChemO.

Revision of the European Detergents Regulation
The revision of the European detergents regulation marks a significant shift for the chemical and fragrance industries.

Extension of data protection for active substances under the Biocidal Products Regulation
Extension of data protection for active substances under the Biocidal Products Regulation. EC agree to extend this period until 2030.

REACH EU Inspections Reveal Infringements in Imported Chemicals and Consumer Products
REACH EU Inspections Reveal Infringements in Imported Chemicals and Consumer Products according to the ECHA’s Enforcement Project.

Major Revision of UK REACH Announced
The UK government has announced a major revision of UK REACH, its domestic REACH regulation. The reform will be completed by the end of 2028.

ECHA Postpones its Opinion on Ethanol to 2026
The biocides sector faces renewed uncertainty following ECHA’s announcement that its BPC has postponed the adoption of its opinion on ethanol.

Ukraine proposes new compliance extensions under UA REACH and UA CLP
Ukraine Proposes New Compliance Extensions Under UA REACH and UA CLP in the last draft resolution published by the Ukrainian Government.

Article 95 Data Protection for Biocides Set to Expire in 2026
The data protection period for certain active substances listed under Article 95 of the Biocidal Products Regulation (BPR) is set to expire.

Brussels puts the brakes on REACH 2.0
Brussels puts the brakes on REACH 2.0: Europe’s chemical regulation overhaul delayed until 2026 asking for more demanding requirements.
SMEs Must Apply for REACH Company Size Validation Before Submitting Dossiers
Under the new rules, small and medium-sized enterprises (SMEs) must apply for REACH company size validation before submitting their dossiers.

Europe Approves the Postponement of CLP Implementation
Europe Approves the Postponement of CLP Implementation. With this change, the regulation will now take effect on 1 January 2028.

Ethanol Reclassification: EU May Label It as Carcinogenic and Reprotoxic
The ethanol reclassification process underway in the EU could bring major regulatory changes for industries relying on ethanol-based products

EU Takes Major Step to Phase Out PFAS in Firefighting Foams
The EC introduced a new restriction on the use of PFAS in firefighting foams to reduce environmental pollution and health risks across the EU

GHS Revision 11 Officially Published: Global Warming contributions new hazard class in chemical regulations
GHS Revision 11 Officially Published: Global Warming contributions are now a formal Hazard Class in Chemical Regulations.

Industry Split Over the Possible Scrapping of the EU’s SCIP Database
Industry Division over the Potential Discontinuation of the EU’s SCIP Database (Substances of Concern in Complex Articles or Products).
Key Updates from the September 2025 RAC and SEAC Meetings
In September 2025, the European Chemicals Agency held important meetings of RAC and SEAC, focusing on several critical regulatory areas.

New Prohibited Substances in EU Cosmetics
New Prohibited Substances in EU Cosmetics. The European Commission has published a new draft amendment to Regulation (EC) 1223/2009.

ECHA issues revised proposal on PFAS restrictions
ECHA issues revised proposal on PFAS restrictions with the inclusion of eight additional sectors not explicitly covered before.

The European Commission announces a review of the BPR to simplify its processes
The European Commission announces a major review of the BPR to simplify its processes due to the number of detected problems in recent years.

Do your biocidal products contain Geraniol? This might interest you
If your biocidal products contain Geraniol, recent regulatory changes in the European Union may significantly affect your business.

The EC Strengthens Cosmetic Safety with a New Regulatory Update
The EC has put forward a draft to update Regulation (EC) No. 1223/2009 on cosmetic products, aiming to further reinforce cosmetic safety

The Omnibus Plan arrives, simplifying processes and pausing time for certain regulatory changes
The European Commission has introduced the Omnibus Plan, a new Action Plan for the Chemical Industry simplifying EU Regulations.

MIT approved under the BPR Regulation for PT6
MIT approved for use in biocidal products of product-type 6 (preservatives for products during storage) according to BPR.

UK to Incorporate New EU CLP Hazard Classes
UK opens consultation to Incorporate New GB CLP Hazard Classes based on EU CLP criteria in Major Chemical Safety Reform.

European Detergents Regulation Update
European Detergents Regulation Update. The EU has taken a decisive step towards greater sustainability and safety with this recent update.

ECHA proposes ten year validity limit on REACH registration dossiers
A central feature of the REACH Regulation revision is the introduction of a ten-year validity limit on REACH registration dossiers.

BIT, DBNPA, and Formic Acid approved in accordance with the BPR Regulation
BIT, DBNPA, and formic acid approved under the BPR Regulation after European Commission decision according to BPR.

Only 7 months remain until the approval of prallethrin as an active biocidal substance for PT18 under the BPR
Only 7 months remain until the approval of prallethrin as an active biocidal substance for PT18 (insecticides) under the BPR.

Updates on REACH Fees for SMEs
Updates on REACH Fees for SMEs. The European Commission has proposed dropping the REACH administrative fees for SMEs.

ECHA Moves to Restrict Chromium VI Substances Across the EU
ECHA to Restrict Chromium VI substances. ECHA has taken a significant decision by proposing an EU-wide restriction on (Cr(VI)) substances.

EU Restricts Microplastics to Combat Environmental Pollution
EU Restricts Microplastics. On October 17, 2023, the European Union implemented Regulation (EU) 2023/2055 under REACH to combat pollution.

CoRAP 2025-2027 to evaluate 28 registered substances
CoRAP 2025-2027 to evaluate 28 registered substances suspected of posing risks to human health or the environment according to REACH.

ECHA announces the next sectors for PFAS restriction assessment
Next sectors for PFAS restriction assessment. RAC and SEAC proposed to include in the PFAS’s review pogram Medical devices and Lubricants.

Ecolabel for Detergents: EU Ecolabel, Guarantee of Sustainability and Efficiency
Ecolabel for Detergents. The EU Ecolabel certifies cleaning products and detergents that meet strict environmental and performance standards

New Packaging Regulation: ECHA will control harmful chemicals in packaging
harmful chemicals in packaging. The Agency will now identify and, when necessary, push for restrictions on these dangerous substances

EU Cosmetics Regulation update
EU Cosmetics Regulation update. The European Commission Launches Evaluation of Cosmetics Safety Rules during the period 2025-2026.

Review of Ethanol: is this biocidal active substance at risk?
Following ECHA’s review of ethanol as a biocidal active substance we need to focus on its approval according to Biocidal Products Regulation.

Official: REACH revision scheduled for the fourth quarter of 2025
REACH revision scheduled: The European Commission has officially confirmed its commitment to overhauling the REACH regulation this year.

The European Commission has proposed a 19.5% increase in REACH fees
The European Commission has proposed a 19.5% increase in REACH fees charged by the European Chemicals Agency for registration purposes.

5 new entries to SVHCs List
ECHA added 5 new entries to SVHCs List. ECHA has recently expanded the SVHCs List, increasing the total number of SVHCs to 247.

REACH Certification for Articles: How to Ensure Your Products Comply with REACH
REACH Certification for Articles: Does your company sell jewelry, appliances, toys, etc.? If so, your business is likely affected by REACH.

40 hazardous chemicals added to PIC
40 hazardous chemicals added to PIC – Are you a chemicals exporter? You can start notifying them now (effective March 1, 2025)

The updated CLP Regulation entered into force on December 10, 2024
ECHA has announced that the updated CLP Regulation about Classification, Labelling and Packaging (CLP) has come into effect on December 10th
November BPC meeting highlights (Biocides, BPR)
November BPC meeting highlights (Biocides, BPR). BPC decided to approve New Biocidal Substances such as MIT and MIT (PT6) and DBNPA (PT11).
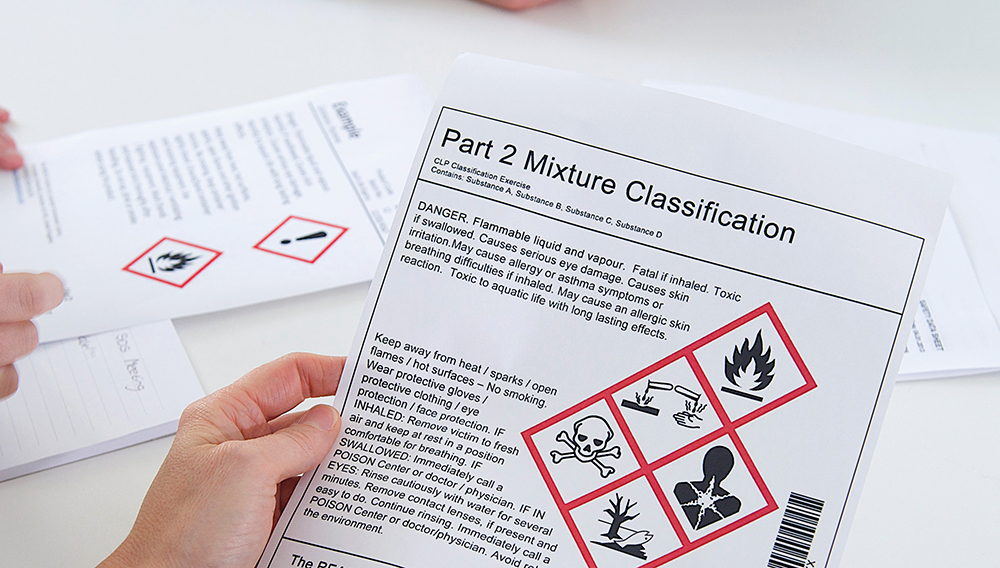
ECHA Inspection: 35% of Safety Data Sheets fail to meet REACH and CLP Standards
ECHA Inspection: 35% of Safety Data Sheets fail to meet REACH and CLP Standards, Safety Data Sheet Compliance it’s a persistent Challenge

Only 7 months remain until the ADBAC/BKC approval for PT2
7 months remain until the ADBAC/BKC approval for PT2 (Disinfectants and algaecides not intended for direct application to humans or animals)

Brazil’s PL 6120/2019 bill approves a REACH-like regulation
Brazil PL 6120/2019 approves new chemical regulation law: A step towards REACH that will help Brazilian companies to ensure chemical safety.

Silver and zinc zeolite approved as an active substance
Silver and zinc zeolite approved as an active substance for use in biocidal products of types 2, 7, and 9, in accordance with BPR

Poison Centre notifications must be in PCN harmonised format as of 1 January 2025
All Poison Centre notifications must be in the harmonised PCN format (Annex VIII of CLP) as of January 1, 2025 according to CLP

The PFAS and talc were the main highlights of the RAC and SEAC committee meeting
Given the importance of PFAS and talc in the daily use of citizens, these were the key points of the RAC and SEAC meeting.

Navigating the BPR Authorization Process: A Comprehensive Guide
Embarking on the BPR authorization process can feel overwhelming Here’s a step-by-step guide to help you understand the requirements.

The Great Britain roster of approved biocidal active substance suppliers is set to lose a staggering 2,700 entries.
Britain is worried about losing up to 2,700 entries on the approved list of biocidal active substance suppliers for the British market.

The European Commission Admits Unrealistic REACH Deadlines
The European Commission Admits Unrealistic REACH Deadlines due to the current characteristics of substances and chemical products.

Ukraine Government Approves Ukraine REACH
On the 23rd of July, the Ukrainian government approved the Ukranian REACH for the safety of chemical products

Approval of prallethrin as an active substance (TP18)
The European Commission publishes the draft approval of prallethrin as an active insecticide substance (TP18)

ECHA scrutinizes more sectors in sweeping PFAS Restriction Proposal
ECHA scrutinizes more sectors in sweeping PFAS Restriction Proposal (F-gases, transport and construction products will be the next)

Clarifying doubts about Same Biocidal Products
This article summarizes the conclusions on the 104th CA meeting about the discussions held about the Same Biocidal Products Regulation.

Chemical hidden dangers: Electronic Cigarettes and Fresh Scents
A New EU Project Targets Mislabeled Mixtures to protect consumers from hidden dangers: Electronic Cigarettes and Fresh Scents

Two-thirds of REACH registrations need updates
REACH registrations need updates. A recent industry review of REACH registrations has revealed significant data gaps.

EU Updates Table of Harmonized Entries in Annex VI to CLP
EU Updates Table of Harmonized Entries in Annex VI to CLP with the Delegated Regulations (EU) 2023/1435 and (EU) 2024/197

UK Streamlines new UK REACH Registration Alternative Transitional Model
UK Streamlines UK REACH Registration with a new Alternative Transitional Model open to consultation on Defra’s website.

Aligning with the European Union: New Standards for Safety Data Sheets in Serbia
Significant step towards harmonization with the EU by adopting a revised format for Safety Data Sheets in Serbia (SDS) for all chemicals.

Brief Explanation: REACH Registration 2 minutes summary
The REACH Registration: REACH stands for Registration, Evaluation, Authorisation and Restriction of Chemicals and came in force in 2006.

The European Parlamient approves CLP Regulation revision
On April 23, the European Parliament approved the revision of CLP to include new CLP hazard categories and other specific requirements.

Endocrine Disruptors: A Brief Introduction
If you are marketing chemicals you have surely heard of endocrine disruptors. Why are they so important? What can we do about them?

Biocide Regulations in Germany: Restrictions on self-service of specific biocidal products
Biocide Regulations in Germany: Restrictions on self-service sale of certain biocidal products such as rodenticides, insectides, etc.

ECHA enforces Poison Centre Notifications for Hazardous Mixtures
ECHA enforces Poison Centre Notifications of hazardous mixtures through inspections that are planned to start in January 2025.

New European Commission Regulation on Allergen Labelling in Detergents
New regulation on allergen labelling in detergents that requires detergent manufacturers to indicate the presence of 81 allergens.

Approved Directive (EU) 2024/825 on Greenwashing
Approved Directive (EU) 2024/825 on Greenwashing: Empowering consumers for the Ecological Transition

BPC February 2024 meeting highlights
BPC February 2024 meeting highlights: positive opinion on the BPR approval of prallethrin and silver zinc zeolite.

What are the different roles of companies in REACH?
The Regulation identifies the different REACH roles and the associated obligations for each one depending on their business activity.

ECHA has already inspected 21% of all REACH registration dossiers submitted
Between 2009 and 2023, the Agency has reviewed approximately 15,000 REACH registration dossiers, representing 21% of all full registrations

ADBAC/BKC approved as an active substance for use in PT2 according to BPR
The approval date for ADBAC/BKC (C12-C16) for use in biocidal products of product-type 2 according to BPR Regulation is 1st July 2025

Material Safety Data Sheets (MSDS)
What are Material Safety Data Sheets? When should they be provided? And… when should they be updated? What is the latest regulatory update?
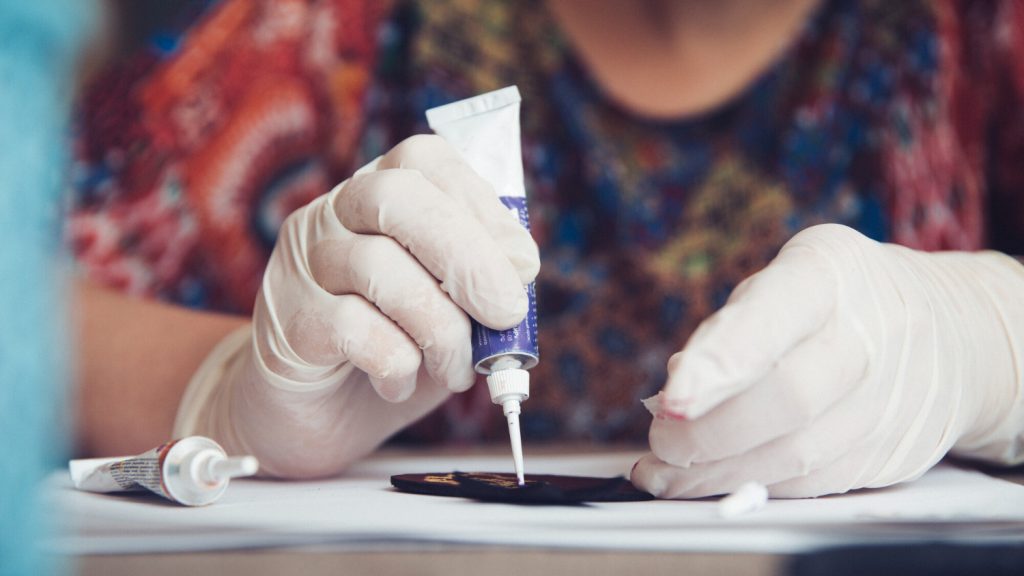
ECHA has added five new substances to the Candidate List of substances of very high concern (SVHCs)
ECHA has added five new substances to the Candidate List of substances of very high concern (SVHCs) due to their associated risks.

Ready to adapt your chemicals to the new CLP hazard classes?
CLP Regulation (31/07/2023) introduces new CLP hazard classes on endocrine disruptors and new deadlines to adapt chemical products

The EU Ecolabel, a guarantee of environmental excellence
The EU Ecolabel is the official voluntary environmental label of the European Union and certifies products with a low environmental impact.

Anticipation Fulfilled: KKDIK Regulation Amendment Officially Announced
Anticipation Fulfilled: KKDIK Regulation Amendment Officially Announced. The amendment was published on December 23, 2023.

Only 1 year left for Chrysanthemum cinerariafolium approval as biocidal active substance (PT18)
Only 1 year left for Chrysanthemum cinerariafolium approval as biocidal active substance as insecticide (PT18) according to BPR.

New agreement for the amendment of the CLP Regulation
Released a provisional agreement to amend the CLP Regulation. This agreement focuses primarily on adapting the regulation to online sales.

UK to loosen chemical registration requirements under UK-REACH
This decision comes after encountering problems with its approach to establishing its own registration requirements under UK-REACH.

One-third of biocidal products fail to meet European biocidal regulations
One-third of biocidal products fail to meet biocidal regulations: EU Biocidal Products Regulation (BPR) and national transitional regulations

Biocidal products in coatings: treated articles or biocides?
Biocidal products in coatings play a crucial role in protection, prevention, and hygiene but what is the applicable regulatory framework?
- Filter by
- Categories
- Tags
- Authors
- Show all
- All
- Biocides
- Carbon Footprint
- CLP
- Cosméticos
- Cosmetics
- Detergents
- Ecolabel
- Environment
- GB CLP
- KKDIK
- Packaging
- PFAS
- PIC
- REACH
- UA-REACH
- UK-REACH
- Waste
- All
- Active substances
- ADBAC
- ADBAC/BKC
- Alkyl (C12-16) dimethylbenzyl ammonium chloride
- Alkyl (C12-16) dimethylbenzylammonium chloride
- allergens
- Annex VIII
- antifouling products
- Antiseptic hand rubs
- Appliances
- Article 95 list
- articles
- BAuA
- Belgium
- Biocidal Products
- biocidal products compliance
- biocidal registration
- Biocide registration
- Biocides
- BIT
- BKC
- BPR
- BPR Application for Authorisation
- BPR Authorisation
- BPR Regulation
- Brazil
- Brexit
- Cadmium
- carbon footprint
- CEFIC
- chemicals
- ChemO
- Chromium VI
- Chrysanthemum cinerariafolium
- cleaning products
- CLP
- CLP Classification
- CLP labeling
- CLP Regulation
- Coatings
- consumers
- CoRAP
- cosmetics
- Cosmetics Regulation
- Data Protection of Active Substances
- DBNPA
- Defra
- detergents
- Detergents Regulation
- Directive (EU) 2024/825
- Directive 98/8/CE
- disinfectants
- ECHA
- Ecolabel
- Ecological Products
- ecological transition
- electronic cigarettes
- endocrine disruptors
- Environment
- Ethanol
- EU Ecolabel
- European Commission
- European Union
- export
- Firefighting Foams
- Forever Chemicals
- Formic Acid
- fragrances
- Fresh Scents
- GB BPR
- GB CLP
- Geraniol
- Germany
- GHS
- GHS revision 11
- Global warming
- Great Britain
- Green Deal
- greenwashing
- Hand sanitizers
- harmful substances
- hazard classes
- hazardous chemicals
- health
- Heavy Metals
- importers
- industry
- insecticides
- Jewellery
- KKDIK
- KKDIK Only Representative
- KKDIK Regulation
- labelling
- labels
- Lead
- lubricants
- manufacturers
- Material Safety Data Sheets
- medical devices
- Microplastics
- MIT
- MSDS
- Nickel
- Omnibus
- Omnibus VI
- online sales
- packaging
- packaging regulation
- packaging waste
- Paints
- personal care
- PFAS
- PIC
- PL 6120/2019
- Poison Centre
- Poison Centre Notification
- polyfluoroalkyl substances
- PPWR
- prallethrin
- Preservatives
- PT13
- PT18
- PT2
- PT6
- pyrethrins
- RAC/SEAC Committee
- REACH
- REACH 2.0
- REACH Annex II
- REACH Annex XVII
- REACH Compliance
- REACH fees
- REACH inspection
- REACH registration
- REACH regulation
- Regulation (EC) No 1223/2009
- Regulation (EC) No 1272/2008
- Regulation (EC) No 1907/2006
- Regulation (EC) No 648/2004
- Regulation (EU) 2023/1435
- Regulation (EU) 2023/2055
- Regulation (EU) 2024/197
- Regulation (EU) no. 649/2012
- Regulation 2020/878
- Regulation EC 1272/2008
- Regulation EU 528/2012
- Regulation UE 528/2012
- restrictions
- rodenticides
- RPC
- safety data sheet
- Same Biocidal Product
- Same Biocide
- SCIP
- SDS
- Serbia
- Silver and zinc zeolite
- silver zinc zeolite
- substance registration
- substances
- substances of very high concern
- Sustainability
- SVHC
- Switzerland
- Talc
- toxicology
- Treated articles
- Turkey
- Turkey REACH
- TurkREACH
- UA REACH
- UK
- UK REACH
- UK REACH Registration
- Ukraine
- Ukraine REACH
- United Kingdom
- waste
- Waste Regulation
- wood preservatives








